GREENVILLE, S.C. — Prisma Health has received emergency use authorization from the U.S. Food and Drug Administration (FDA) for a unique ventilator expansion device designed by Prisma Health physicians.
The new device allows a single ventilator to support up to four patients during times of acute equipment shortages such as the current COVID-19 pandemic.
Produced using 3D printing technology, the VESper ventilator expansion device is developed with material already in use for medical devices and produced at minimal cost.

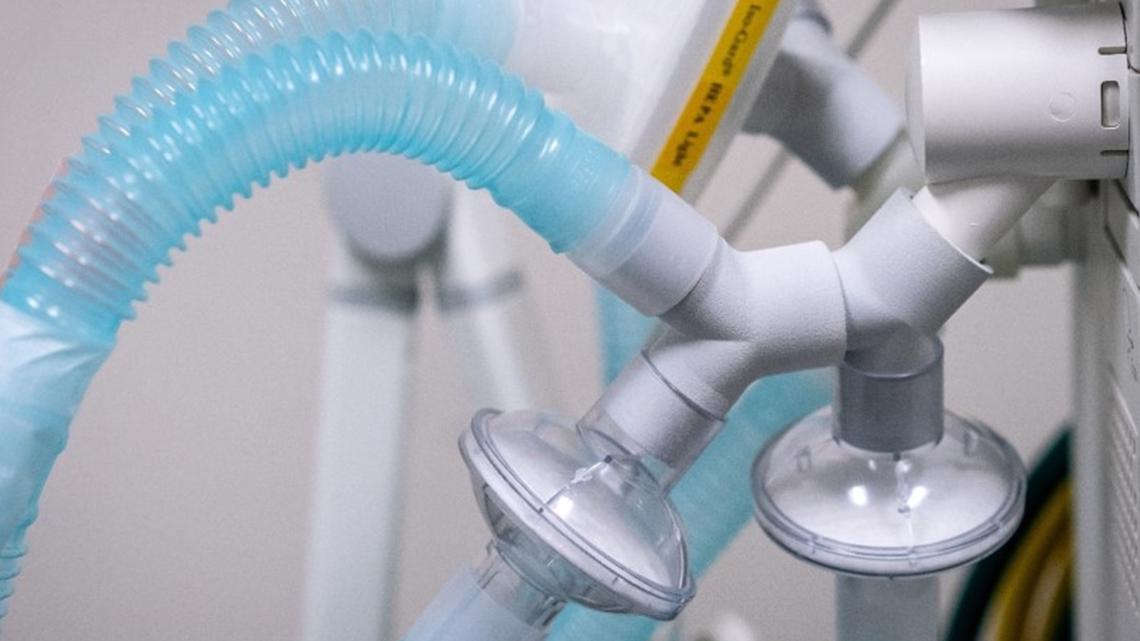
Prisma Health experts are working with national COVID-19 teams, who have no more ventilator capacity and who can make emergency use of the prototype.
The emergency use authorization can offer critical care patients access to a medical device that has not gone through normal FDA approval; this is used when no comparable or satisfactory alternative options are available.
“The VESper device can be lifesaving when the number of critically ill patients requiring breathing support is greater than the number of available ventilators," said Peter Tilkemeier, M.D., chair of the Department of Medicine at Prisma Health-Upstate. "A number of U.S. hospitals are likely to begin experiencing this with COVID-19.”
Officials say a Prisma Health emergency medicine physician realized the opportunity of using a single machine to breathe for multiple patients. Working collaboratively with her husband, a software engineer and with a Prisma Health pulmonary critical care physician, this team began developing specifications for a “Y” splitter tubing that could be easily produced, allow for appropriate filtering of bacteria and viruses in the ventilator tubing, be strong and impact resistant, and would not impact the care of other patients connected to the same machine.


Drawing on the strength of Prisma Health’s existing academic partnerships, specifications were sent to engineers at Clemson University and University of South Carolina for 3D materials testing and printing of prototypes. The team began working to secure FDA approval and collaborations with private sector businesses came together within a matter of days.
Hospitals can begin to apply to receive the free source code and printing specifications for the device immediately by registering on Prisma Health’s Website www.prismahealth.org/VESper for their use in printing the device.
Prisma Health says it is collaborating with major companies such as HP Inc. and its Digital Manufacturing Network to quickly scale 3D production of validated parts for distribution in areas of greatest need and areas with the potential to exceed their ventilator capacity in the near future, such as COVID-19 “hot spots” designated by the Federal Emergency Management Agency (FEMA).
Currently Prisma Health and South Carolina hospitals have enough ventilators available for patients, according to Prisma Health.
“I am so proud of the creativity and perseverance of our clinical team who came together to develop a potentially life-saving solution at a critical time for our country, our communities and our patients," said Mark O’Halla, president and chief executive officer of Prisma Health.

