COLUMBIA, S.C. — The Medical University of South Carolina is looking for volunteers for a clinical trial to test three common drugs in the fight against COVID-19.
One of the drugs is ivermectin.
“It is such a cool trial. You don’t even have to go to a doctor’s office to be part of this trial. It’s completely remote,” said Leslie Lenert,M.D., director of the Biomedical Informatics Center at the Medical University of South Carolina. Participants do not have to live in Charleston or near MUSC to participate.
Called ACTIV-6, the trial is led by the Duke Clinical Research Institute in North Carolina and covers several states. Lenert is the principal investigator for MUSC. Rami Zebian, M.D., chief medical officer of MUSC Health-Florence Division, serves as clinical co-investigator for the Florence and Marion hospitals.
"We have seen a lot of misinformation out there, and unfortunately, some patients have received non-FDA approved medications outside of a clinical trial. That is not a safe practice, and we have seen many reports of toxicity – and we have many unanswered questions," Zebian said. "I strongly believe that a clinical trial helps us answer those questions and offers possible treatment options in a safe and controlled manner.”
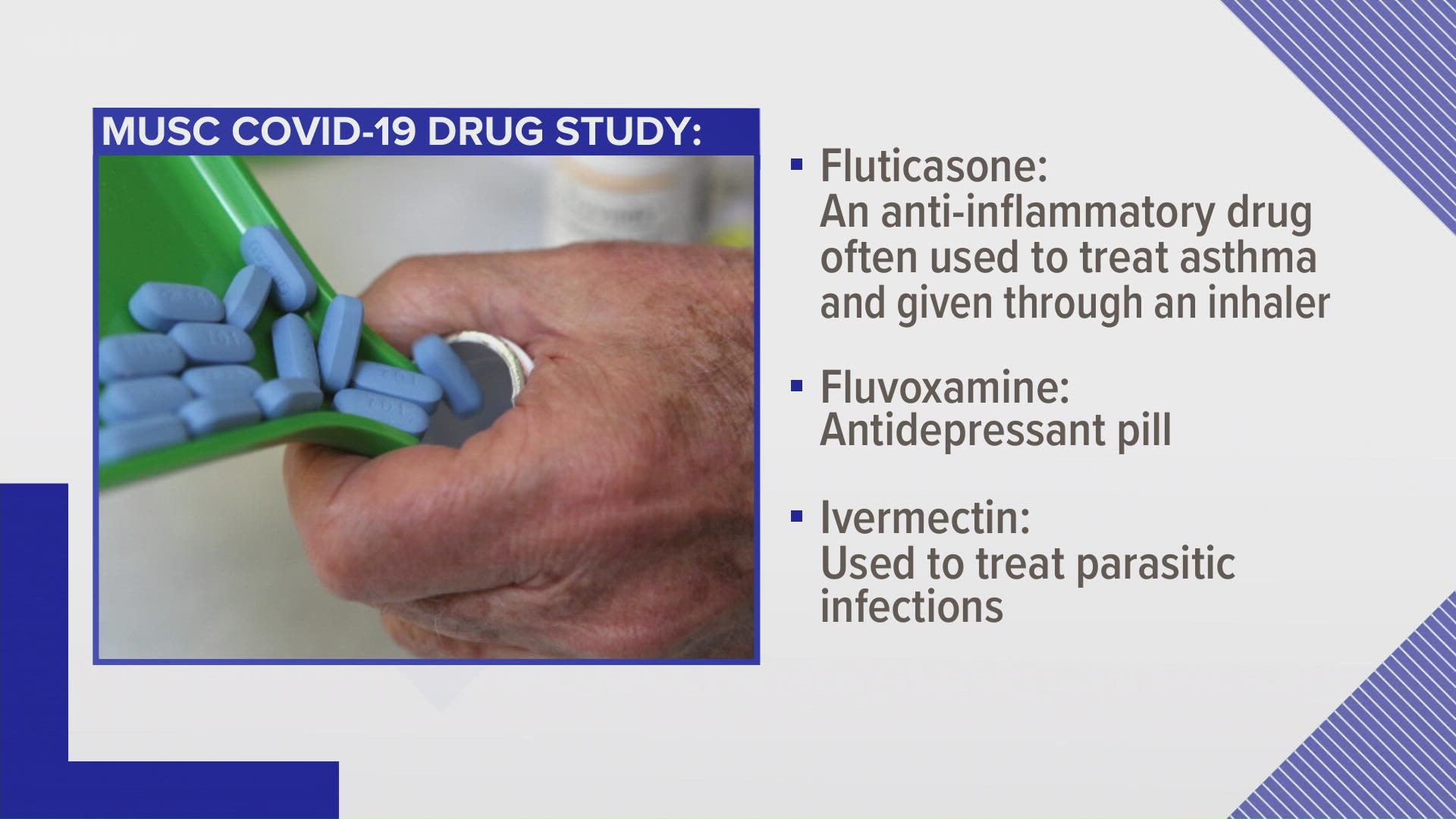
The trial is testing three drugs that could help people with mild to moderate illness who are recovering at home. The study drugs are fluticasone, a corticosteroid often used for asthma or chronic obstructive pulmonary disease that is delivered via inhaler; fluvoxamine, an antidepressant in pill form; and ivermectin, a drug that is used to treat parasitic infections and that has been in the news quite a lot lately.
“Everybody's heard about ivermectin,” said Elizabeth Szwast, the study coordinator. “It’s a really hot topic right now, and this is a safe environment to see whether it’s effective to reduce COVID-19 symptoms.”
Lenert said that volunteering for the study can help to settle the question of ivermectin’s effectiveness.
“If you believe in it – get into a clinical trial. If you don’t believe in it – get into a clinical trial. Let’s get the answer out, and let’s stop fighting each other over it,” he said.
People interested in the trial will have the option to choose which study arm they would like to be a part of.
Once someone is accepted into the trial, the central pharmacies mail the study drug and a pulse oximeter to the patient. The patient takes the study medication as directed, fills out daily surveys online and responds to phone call questionnaires on days 14 and 28. After 90 days, the participants each receive a $100 Amazon gift card as a thank-you gesture for time and participation in the clinical trial.
Anyone interested in participating in the study can call 843-792-4675 or visit activ6study.org.
To be eligible for the study, patients must:
- Be over 30 years old.
- Have had a positive COVID-19 test within the past 10 days.
- Be experiencing at least two of the following symptoms: feeling tired, trouble breathing, fever, cough, upset stomach, vomiting, diarrhea, body aches or chills, headache, sore throat, stuffy nose or new loss of taste or smell.