CHARLESTON, S.C. — The Medical University of South Carolina (MUSC) is performing clinical trials in Charleston of three potential vaccine options by companies AstraZeneca, Janssen and Novavax in hopes of boosting the number of vaccines available.
According to university officials, the trials are each randomized with some people receiving the vaccine while others get a placebo.
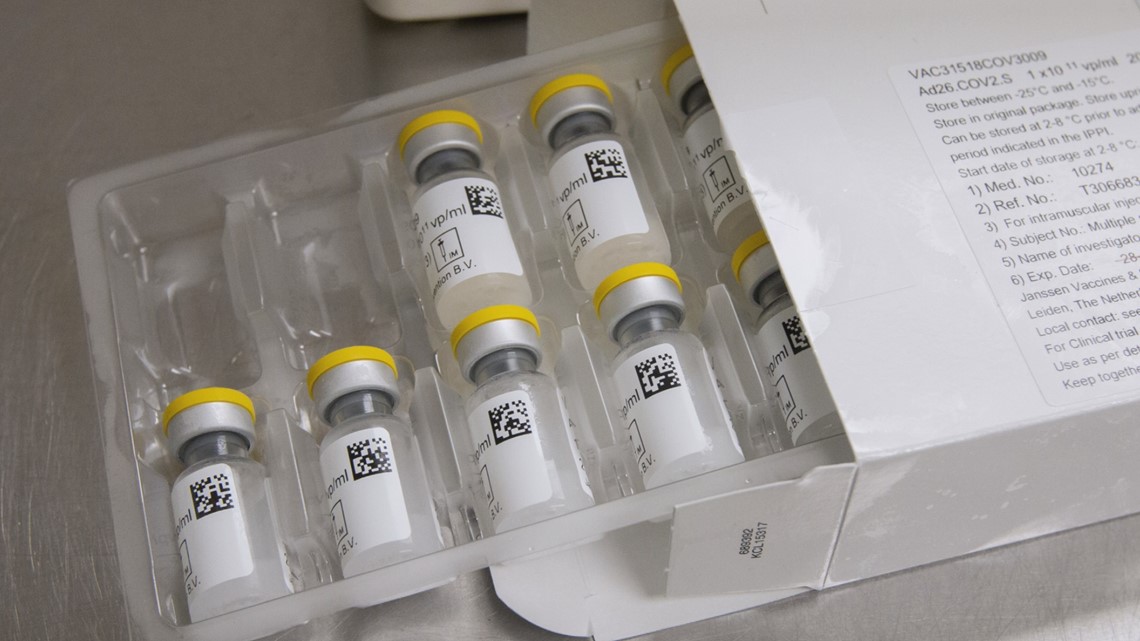
AstraZeneca and Novavax both require two shots. The Janssen vaccine, right now, only requires one, but they’re studying a second option where two doses would be needed to see if that improves effectiveness.
Dr. Patrick Flume with MUSC said the data they collect will join that of other health facilities around the country to determine if the vaccines can help in the fight against the virus.

“The studies that we are doing are enrolling adult subjects,” Dr. Flume said. “To show that it works, ideally what you’d like to know is if I’m going to use the vaccine in young people and old people, men and women, all races, I want to have information that shows that it’s effective and safe in all of those groups.”
Dr. Flume added that they’re still seeking adult (18 and up) participants for the Janssen trial, though those interested would have to travel to Charleston to participate. Click here to learn how to participate.
Currently the Pfizer-BioNTech and the Moderna vaccines are the only vaccines that have gotten emergency approval in the United States. Johnson & Johnson's vaccine is set to go before the FDA before the end of the month.